Lunesta
http://www.medicinenet.com/eszopiclone/article.htm
GENERIC NAME: eszopiclone
BRAND NAME: Lunesta (formerly known as Estorra)
DRUG CLASS AND MECHANISM: Eszopiclone is a non-benzodiazepine, oral, sedative drug ("sleeping pill") that is used for treating insomnia. According to the National Institutes of Health, more than 50 million Americans suffer from insomnia. Symptoms of insomnia include difficulty falling asleep, awakening frequently during the night, waking up too early, an inability to fall back to sleep, or awakening in the morning not feeling refreshed. Most drugs that have been used to treat insomnia are benzodiazepines, for example, flurazepam (Dalmane), lorazepam (Ativan), triazolam (Halcion), and temazepam (Restoril). Zolpidem (Ambien) was the first non-benzodiazepine approved for insomnia in over 20 years. Eszopiclone is unique in that it is the only drug used for insomnia that has been shown to be safe and effective for up to six months. Eszopiclone was approved by the FDA in December, 2004.
GENERIC: No
PRESCRIPTION: Yes
PREPARATIONS: Tablets of 1, 2, and 3 mg
STORAGE: Tablets should be stored at room temperature, 15-30 °C (59-86 °F).
PRESCRIBED FOR: Eszopiclone is used for the treatment of insomnia characterized by difficulty falling asleep and/or difficulty maintaining sleep during the night and early morning.
DOSING: The usual dose to improve or maintain sleep in most adults is 2 or 3 mg. Persons over the age of 65 years usually are treated with 1 or 2 mg. Eszopiclone should be taken immediately before going to bed since the onset of sedation may occur as rapidly as 10 minutes. It should be taken only by individuals who intend to sleep for at least 8 hours since its effects may last up to six hours.
DRUG INTERACTIONS: Alcohol (which causes sedation) and drugs that have sedating effects should not be used with eszopiclone since their sedating effects, when added to those of eszopiclone, may cause excessive sedation.
PREGNANCY: Eszopiclone should not be used during pregnancy.
NURSING MOTHERS: It is not known whether eszopiclone is excreted in human breast milk. Because many medicines are excreted in breast milk and because the effect of eszopiclone on infants has not been studied, women should not breast feed while taking eszopiclone.
SIDE EFFECTS: Patients taking eszopiclone or any other sedative drug may develop dependence on the drug for sleep and experience withdrawal symptoms when the drug is discontinued. The most common side effects of eszopiclone are dizziness and loss of coordination.
---------------------------------------------------------------
http://www.rxlist.com/cgi/generic3/lunesta.htm
LUNESTA™ (eszopiclone) TABLETS 1 mg, 2 mg, 3 mg
DESCRIPTION
LUNESTA (eszopiclone) is a nonbenzodiazepine hypnotic agent that is a pyrrolopyrazine derivative of the cyclopyrrolone class. The chemical name of eszopiclone is (+)-(5S)-6-(chloropyridin-2-yl)-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazin-5-yl 4-methyl- piperazine-1-carboxylate. Its molecular weight is 388.81, and its empirical formula is C17H17ClN6O3.
Eszopiclone has a single chiral center with an (S)-configuration. It has the following chemical structure:
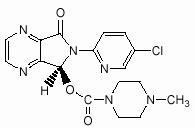
Eszopiclone is a white to light-yellow crystalline solid. Eszopiclone is very slightly soluble in water, slightly soluble in ethanol, and soluble in phosphate buffer (pH 3.2).
Eszopiclone is formulated as film-coated tablets for oral administration. LUNESTA tablets contain 1 mg, 2 mg, or 3 mg eszopiclone and the following inactive ingredients: calcium phosphate, colloidal silicon dioxide, croscarmellose sodium, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, and triacetin. In addition, both the 1 mg and 3 mg tablets contain FD&C Blue #2.
-----------------------------------------------
http://www.drugs.com/lunesta.html
Lunesta
eszopiclone (ezz sop e clone)
What is the most important information I should know about Lunesta?
•
Use caution when driving, operating machinery, or performing other hazardous activities. Lunesta will cause drowsiness and may cause dizziness. If you experience drowsiness or dizziness, avoid these activities. Lunesta should be taken just before bedtime. You may experience some carryover effects the next day.
•
Do not drink alcohol while taking Lunesta. Alcohol will increase drowsiness and may increase dizziness while you are taking Lunesta, which could be dangerous.
•
Do not stop taking Lunesta suddenly if you have been taking it for more than 1 or 2 weeks. This may cause withdrawal symptoms and make you uncomfortable. Talk to your doctor if you need to stop treatment with Lunesta.
What is Lunesta?
•
Lunesta is in a class of drugs called sedative/hypnotics or sleep medications. Lunesta affects chemicals in your brain that may affect sleep.
•
Lunesta induces sleep and causes relaxation. It is used to treat insomnia.
•
Lunesta may also be used for purposes other than those listed in this medication guide.
What should I discuss with my healthcare provider before taking Lunesta?
•
Before taking this medication, tell your doctor if you
·
have liver disease;
·
have asthma, bronchitis, emphysema, or another respiratory disease; or
·
are depressed or have suicidal thoughts.
•
You may not be able to take Lunesta, or you may require a lower dose or special monitoring during treatment if you have any of the conditions listed above.
•
Lunesta may cause memory loss or "amnesia" where a person may not remember what has happened for several hours after taking the medication. Since Lunesta is typically taken just prior to falling asleep for the night, this is generally not a problem. However, this could be a problem if Lunesta is taken while traveling, such as during an airplane flight, and the person wakes up before the effects of the medication are gone. Lunesta should only be taken if you are able to get a full night's sleep (8 or more hours) before you must be active again.
•
Be aware that you may have more sleeping problems the first night or two after you stop taking Lunesta.
•
Lunesta is in the FDA pregnancy category C. This means that it is unknown whether Lunesta will harm an unborn baby. Do not take Lunesta without first talking to your doctor if you are pregnant.
•
Lunesta passes into breast milk and may affect a nursing baby. Do not take this medication without first talking to your doctor if you are breast-feeding a baby.
•
If you are over 65 years of age, you may be more likely to experience side effects from Lunesta. You may require a lower dose of this medication.
How should I take Lunesta?
•
Take Lunesta exactly as directed by your doctor. If you do not understand these directions, ask your pharmacist, nurse, or doctor to explain them to you.
•
Take each dose with a full glass of water.
•
Take Lunesta just before you go to bed. It will make you drowsy, and you could fall and hurt yourself if you take your dose before you are ready for sleep.
•
You can take Lunesta with or without food but for Lunesta to work best, do not take it with or immediately after a high-fat, heavy meal.
•
Do not crush or chew the tablets. Take each tablet whole.
•
Take Lunesta only if you are able to get a full night's sleep (8 or more hours) before you must be active again.
•
Do not take more of this medication than is prescribed for you.
•
Do not stop taking Lunesta suddenly if you have been taking it for more than 1 or 2 weeks. This may cause withdrawal symptoms and make you uncomfortable. Talk to your doctor if you need to stop treatment with Lunesta.
•
Store Lunesta at room temperature away from moisture and heat.
What happens if I miss a dose?
•
Since Lunesta is usually taken only if you need it to help you sleep, missing a dose will not cause any problems. Take the missed dose only if you can be sure that you will get 8 full hours of sleep after the dose. If you do not sleep 8 full hours, you may experience carryover effects from Lunesta after you wake up.
What happens if I overdose?
•
Seek emergency medical attention.
•
Symptoms of a Lunesta overdose may include sleepiness, confusion, dizziness, difficult or slow breathing, and unconsciousness.
What should I avoid while taking Lunesta?
•
Use caution when driving, operating machinery, or performing other hazardous activities. Lunesta will cause drowsiness and may cause dizziness. If you experience drowsiness or dizziness, avoid these activities. Lunesta should be taken just before bedtime. You may experience some carryover effects the next day.
•
Do not drink alcohol while taking Lunesta. Alcohol will increase drowsiness and may increase dizziness while you are taking Lunesta, which could be dangerous.
•
Avoid other sedatives, sleeping pills, and tranquilizers, including over-the-counter preparations. They should not be used while you are taking Lunesta unless your doctor directs otherwise.
What are the possible side effects of Lunesta?
•
If you experience any of the following serious side effects, stop taking Lunesta and seek emergency medical attention:
·
an allergic reaction (difficulty breathing; closing of your throat; swelling of your lips, face, or tongue; hives).
•
Contact your doctor if you experience
·
daytime drowsiness, dizziness, or clumsiness;
·
more outgoing or aggressive behavior than normal;
·
confusion;
·
strange behavior;
·
memory loss problems;
·
agitation;
·
worsening of depression;
·
hallucinations; or
·
new feelings of depression.
•
Other, less serious side effects may be more likely to occur, such as headache and unpleasant taste. If these become bothersome, contact your doctor.
•
A problem that may occur when sleep medicines are stopped is known as "rebound insomnia." This means that a person may have more trouble sleeping the first few nights after the medicine is stopped than before starting the medicine. If you should experience rebound insomnia, do not get discouraged. This problem usually goes away on its own after 1 or 2 nights.
•
Lunesta may be habit forming. Stopping this medication suddenly can cause withdrawal effects if you have taken it continuously for several weeks. Talk to your doctor about the safe use of this medication.
•
Side effects other than those listed here may also occur. Talk to your doctor about any side effect that seems unusual or that is especially bothersome.
What other drugs will affect Lunesta?
•
Many drugs may affect the way that Lunesta is metabolized ("broken down") in the body, leading to higher or lower than expected levels of the medication in the blood. Talk to your doctor before taking any other medicines during treatment with Lunesta.
•
Lunesta may increase the effects of other drugs that cause drowsiness, including antidepressants, alcohol, antihistamines, other sedatives (used to treat insomnia), pain relievers, anxiety medicines, and muscle relaxants. Tell your doctor about all medicines that you are taking, and do not take any medicine unless your doctor approves.
•
Drugs other than those listed here may also interact with Lunesta. Talk to your doctor and pharmacist before taking any prescription or over-the-counter medicines including vitamins, minerals, and herbal products.
Where can I get more information?
•
Your pharmacist has additional information about Lunesta written for health professionals that you may read.
----------------------------------------------------
http://www.immunesupport.com/library/showarticle.cfm/ID/6224
Lunesta: You May Want to Sleep on It
ImmuneSupport.com01-20-2005 Enter the Next 'Miracle' Drug: a Sleeping Pill You Can Take Long-Term. Ads for Lunesta Won't Likely Give That Theme a Rest. This Time Will Consumers Be More Leery?
By Sandra G. BoodmanWashington Post Staff WriterTuesday, January 18, 2005; Page HE01
It sounds like an insomniac's dream: a sleeping pill that can be taken for weeks or even months at a time, without the risk of addiction or morning-after grogginess.
In the next several weeks consumers will see splashy print and television ads touting the lyrically named Lunesta, which was approved last month by the U.S. Food and Drug Administration (FDA). Unlike other sleeping pills, including market leader Ambien, which are not supposed to be taken for longer than 10 days at a time, Lunesta has no FDA recommended time limit.
Since the drug's official launch last week, Sepracor, its Massachusetts-based manufacturer, has deployed 1,250 salespeople to the offices of primary care physicians -- the doctors most patients consult for sleep problems -- as well as psychiatrists and hospitals. The aim, said David P. Southwell, Sepracor's chief financial officer, is to persuade them of Lunesta's superiority in treating insomnia, an extremely common problem that regularly affects more than half of American adults and that, in Southwell's view, is "under-recognized and under-treated."
Some sleep specialists question the wisdom of using a sleeping pill for weeks or months on end, particularly when it is a new drug approved after six months of testing in 2,700 patients.
They cite the fresh examples of the increased risk of heart attack and stroke from arthritis pain relievers Vioxx, Celebrex and Bextra, the dangers of which emerged after millions of patients started taking them.
Because insomnia is so common -- Ambien is the nation's 12th-best-selling prescription medication, according to IMS Health -- and because Lunesta will be aggressively marketed, some sleep specialists emphasize the importance of non-drug remedies.
"The best thing to do is to avoid getting into a situation where you need a medication long-term," said Northern Virginia neurologist John W. Cochran. For a small group of patients who have been adequately screened to rule out underlying physical or psychiatric problems -- such as depression or anxiety -- that might cause insomnia, long-term use of a sleeping medication may be indicated, he said.
To treat insomnia many sleep specialists, including Cochran, recommend behavioral strategies that fall under the schoolmarmish rubric "sleep hygiene." They include relaxation techniques as well as avoidance of caffeine, alcohol and large meals before bedtime. Sleeping pills are often used short-term, to break the cycle of sleeplessness and the anxiety it causes.
Cochran said he worries that many consumers, eager for a speedy and easy remedy, will get a prescription for Lunesta from a primary care doctor who has neither the time nor the training to suggest behavioral techniques or conduct a comprehensive evaluation.
"That means some doctors will give it to patients who will stay on it forever," he said.
Symptoms of insomnia -- difficulty falling or staying asleep -- are extremely common. A 2002 poll by the National Sleep Foundation found that 58 percent of adults experience them a few times each week and one-third have nightly symptoms. The problem is more common among frequent travelers, shift workers, women and the elderly. Another study found that about half of people with insomnia suffer from an underlying medical problem, such as depression, anxiety or chronic pain.
In about 15 percent of cases, sleep specialists say, chronic insomnia has no apparent underlying cause. Gregg D. Jacobs, a sleep specialist at Beth Israel Deaconess Medical Center in Boston, said that long-term use of a sleeping pill is inappropriate for most patients because cognitive behavioral therapy works better than drugs in overcoming insomnia.
Sleeping pills, Jacobs said, are typically prescribed for brief periods. Some medicines used for sleep are habit-forming, such as a class of drugs known as benzodiazepines, which include Valium and Xanax. Doctors worry that long-term use of non-narcotic medications such as Lunesta can create psychological dependence that results when patients fear they can't fall asleep or stay asleep without them.
The other problem, said Jacobs, an assistant professor of psychiatry at Harvard Medical School, is that the long-term effects of Lunesta are unknown.
"Lunesta is like every drug approved by the FDA," said Jacobs. "We don't know what the long-term side effects are" or all the negative side effects seen during the clinical trials. "The message is: Buyer beware."
Sepracor's Southwell said that the drug, known generically as eszopiclone, is closely related to zopiclone, a sleep drug widely used in Europe and Canada for 20 years. Zopiclone, he said, has a good safety record. "If there were a safety defect, one would think they would have seen it by now," he said.
But Southwell acknowledged that oversight of prescription drugs varies from country to country in Western Europe. And some sleep specialists say that the potential market for Lunesta may be larger in the United States because of the aggressive marketing of drugs to consumers.
In the clinical trials of Lunesta, the most common side effects reported to the FDA were headache, an unpleasant taste and dizziness. When they first start taking the drug users are advised to "use extreme care when doing anything that requires complete alertness" such as driving, piloting an airplane or using heavy machinery.
Terri Bagley said she has been counting the days until Lunesta hit the market so she could call her doctor for a prescription.
Bagley, 43, who operates a housecleaning business in Pelham, N.C., said she has battled chronic insomnia for more than 20 years. She said it routinely takes her two hours to fall asleep at night, and she usually awakens four or five times each night, snagging a total of about four hours sleep and feeling perpetually exhausted during the day. None of the host of prescription or nonprescription drugs she tried made much difference, she said, and doctors ruled out underlying psychiatric or medical problems that might be causing her insomnia.
Then Bagley said, she enrolled in the clinical trial of Lunesta conducted at the Duke University Sleep Disorders Center. She said she is certain she got the drug rather than a placebo.
"I've never in my life slept that well or felt that good," Bagley said, adding that during the three months she took the drug she slept about seven hours per night with fewer and shorter awakenings.
Andrew D. Krystal, director of Duke's sleep center and one of the principal investigators of Lunesta, said that another advantage of the drug is that patients did not build up a tolerance to it. That is a common side effect of benzodiazepines, which typically require progressively larger doses to achieve the same effect.
Krystal, who has worked as a consultant for Sepracor, said while behavioral treatments are effective for some people, others have more intractable insomnia and need medication "which should be taken at the lowest possible dose for the shortest duration."
Whether Lunesta works better than its competitors remains to be seen. Chevy Chase sleep specialist Helene Emsellem said no large studies have compared Lunesta with other drugs.
"I think Lunesta clearly fills a needed niche in selected patients," said Emsellem, an associate clinical professor of neurology at George Washington University School of Medicine, who was involved in the clinical trials of the drug but said she has no other financial relationship with Sepracor. "But it's important for people to realize that insomnia is often a symptom and not a disease, and to sort out the problems."
Source and © 2005 The Washington Post Company.
------------------------------------------------------------------------
http://www.rxlist.com/cgi/generic3/lunesta_ids.htm
INDICATIONS AND USAGE
LUNESTA is indicated for the treatment of insomnia. In controlled outpatient and sleep laboratory studies, LUNESTA administered at bedtime decreased sleep latency and improved sleep maintenance.
DOSAGE AND ADMINISTRATION
The dose of LUNESTA should be individualized. The recommended starting dose for LUNESTA for most non-elderly adults is 2 mg immediately before bedtime. Dosing can be initiated at or raised to 3 mg if clinically indicated, since 3 mg is more effective for sleep maintenance (see PRECAUTIONS).
The recommended starting dose of LUNESTA for elderly patients whose primary complaint is difficulty falling asleep is 1 mg immediately before bedtime. In these patients, the dose may be increased to 2 mg if clinically indicated. For elderly patients whose primary complaint is difficulty staying asleep, the recommended dose is 2 mg immediately before bedtime (see PRECAUTIONS).
Taking LUNESTA with or immediately after a heavy, high-fat meal results in slower absorption and would be expected to reduce the effect of LUNESTA on sleep latency (see Pharmacokinetics under CLINICAL PHARMACOLOGY).
Special Populations
Hepatic
The starting dose of LUNESTA should be 1 mg in patients with severe hepatic impairment. LUNESTA should be used with caution in these patients.
Coadministration With CYP3A4 Inhibitors
The starting dose of LUNESTA should not exceed 1 mg in patients coadministered LUNESTA with potent CYP3A4 inhibitors. If needed, the dose can be raised to 2 mg.
HOW SUPPLIED
LUNESTA 3 mg tablets are round, dark blue, film-coated, and identified with debossed markings of S193 on one side, and are supplied as:
NDC 63402-193-10
bottle of 100 tablets
NDC 63402-193-09
carton of 90 tablets
LUNESTA 2 mg tablets are round, white, film-coated, and identified with debossed markings of S191 on one side, and are supplied as:
NDC 63402-191-10
bottle of 100 tablets
NDC 63402-191-09
carton of 90 tablets
LUNESTA 1 mg tablets are round, light blue, film-coated, and identified with debossed markings of S190 on one side, and are supplied as:
NDC 63402-190-10
bottle of 100 tablets
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Manufactured for: Sepracor Inc., Marlborough, MA 01752 USA by Patheon Inc., Mississauga, Ontario L5N 7K9 Canada, For customer service, call 1-888-394-7377. To report adverse events, call 1-877-737-7226. For medical information, call 1-800-739-0565., December 2004 IN-5169/S
--------------------------------------
http://www.sepracor.com/therap/lunesta.html
LUNESTATM (eszopiclone)
On December 15, 2004, the U.S. Food and Drug Administration (FDA) approved the New Drug Application (NDA) for LUNESTATM brand eszopiclone, formerly referred to as ESTORRA, 1 mg, 2 mg, and 3 mg tablets for the treatment of insomnia. LUNESTA brand eszopiclone is expected to be made available by prescription within the first quarter of 2005. Insomnia can include difficulty falling asleep as well as difficulty maintaining sleep through the night. The recommended dosing to improve sleep onset and/or maintenance is 2 mg or 3 mg for adult patients (18 to 64) and 2 mg for older adult patients (ages 65 and older). The 1 mg dose is for sleep onset in older adult patients whose primary complaint is difficulty falling asleep.
The NDA for LUNESTA contained data from 24 clinical trials, which included more than 2,700 adult and elderly patients, and more than 60 preclinical studies. Results of six randomized, placebo-controlled Phase III studies for the treatment of chronic or transient insomnia in both adult and older patients (ages 65 and older) were included as part of the NDA package. Data from a landmark, long-term (six-month), double-blind, placebo-controlled safety and efficacy study in 788 patients were reviewed by the FDA as part of the NDA submission for eszopiclone and served as a basis for the FDA's decision to not limit LUNESTA's indication to short-term use. Sepracor's six-month study was the first of its kind for a prescription non-benzodiazepine for the treatment of insomnia. The results of this study were published in the November 2003 issue of the journal SLEEP.
Clinical data on LUNESTA has been presented at various medical society meetings in 2004, including the annual meetings of the American College of Obstetricians and Gynecologists (ACOG), the American Psychiatric Association (APA), and the Associated Professional Sleep Societies (APSS). For further information about the clinical data presented at medical meetings in 2004, please click here or refer to Sepracor's press releases dated, May 6, May 13, and June 8-10, 2004, located on this web site.
Sepracor continues to study LUNESTA in adult and older adult patients in a comprehensive Phase IIIB/IV program. The preliminary results of a Phase IIIB/IV, 545-patient, double-blind, placebo-controlled, ten-week study evaluating the efficacy and safety of LUNESTA in patients with insomnia and co-existing Major Depressive Disorder (MDD) have been completed. Sepracor has submitted results of this study to be considered by the American Psychiatric Association and the Associated Professional Sleep Societies for presentation at their respective scientific meetings in May and June of 2005.
Additional Phase IIIB/IV studies of LUNESTA are nearing completion. These studies include the evaluation of LUNESTA for the treatment of insomnia in women experiencing the hormonal changes associated with perimenopause, in patients experiencing pain associated with rheumatoid arthritis, and a six-month, double-blind, placebo-controlled study in patients with chronic insomnia.
Sepracor currently anticipates that LUNESTA will be available to patients nationwide within the first quarter of 2005.
Insomnia
An estimated 100 million adult Americans suffer from either chronic or occasional insomnia*. Symptoms of insomnia include difficulty falling asleep, awakening frequently during the night, waking up too early, an inability to fall back to sleep, or awakening feeling unrefreshed. Insomnia can be a serious condition. If left untreated, it may become progressively worse and in turn, potentially affect a person's emotional, mental and physical health.
The U.S. market for prescription sleep products, not including off-label (not indicated for the treatment of insomnia) use of central nervous system (CNS) agents for the treatment of insomnia, was approximately $2.1 billion between November 2003 and October 2004, representing a 20 percent increase over the same period the previous year, according to IMS Health Information.
*Extrapolated to current population from 2000 census based on Ancoli-Israel et al. SLEEP. 1999; 22 (suppl 2):S347-S353.
Important Safety Information
It is important to note that because sleep disturbances may be caused by underlying physical and/or psychiatric disorders, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7-10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Patients should only take LUNESTA when they are prepared to get a full night of sleep. Until they know how they will react to LUNESTA, patients should not drive or operate machinery.






1 Comments:
I have the same taste and feel like I'm being poisoned... It does make me sleep but the side efeects are unreal!!
Post a Comment
<< Home